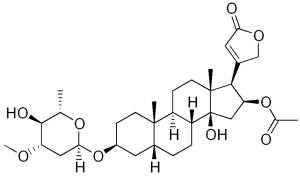
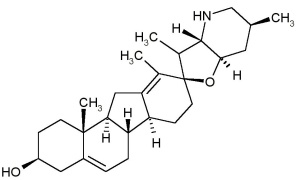
A while ago I posted about oleander and the structural similarities between the oleander cardiac glycoside oleandrin and the foxglove cardiac glycoside digoxin. But foxgloves express enough biologically useful (and harmful) molecules that they’re worth showcasing. Plus they’re such nice eye-candy!
Gloves that pack a punch
The various members of the Digitalis genus, which include the gardener’s favorite foxglove Digitalis purpurea and a range of other Digitalis species, are favored ornamental plants for their tall showy flower spikes and bright colors. A disputed but appealing origin for the name was advanced by William Henry Fox Talbot, who proposed that the whimsical ye Olde English people imagined fairies wearing the deep cone-shaped flowers for gloves, and called them folks’ gloves. Very cute. But given that fairies were famous not only for being adorable but also for other light-hearted mischiefs like stealing babies and poisoning livestock, it’s perhaps fitting that their pleasing-to-look-at gloves come barbed with a heavy dose of serious poison.

Enough folk's gloves for a children's book full of fairies. Digitalis purpurea, by Ferdinand Bauer.
Foxgloves are poisonous because they contain two cardiac glycosides, digoxin and digitoxin, which are found in all parts of the foxglove plant but are most concentrated in the leaves. There are a few ways to poison yourself with foxgloves by mistake: the flowers have some appealing similarities to honeysuckle, which might lead the unwary to try to suck nectar from them. Because the plant is still poisonous when dry, a hapless gardener might inadvertently inhale foxglove plant matter when digging or replanting near an old foxglove bed. The leaves of foxglove (especially before it flowers) resemble and have sometimes been mistaken for comfrey, which is benign and a common basis for tea (here’s an article about several people poisoned this way). Never fear though, ehow has a handy how-to on distinguishing comfrey from foxglove leaves; the clearest difference might be that foxglove leaves are finely toothed while comfrey’s are smooth.

Leaf of comfrey, Symphytum sps. Good for tea, beloved by herbalists, and pretty easy to confuse with foxglove leaves, pictured in the illustration above. Photo courtesy of Heather at ahandmadelife.blogspot.com, which is also a pretty nifty blog.
Finally, one of the side effects of digitalis poisoning is strong hallucinations, so there may be a handful of people out there ingesting it deliberately…but I doubt the visuals are worth the heart arrhythmias, severe nausea, fainting, coma and possible death that come with them.
Extra nitty-gritty: Digoxin as heart medicine
Cardiac glycosides like digoxin and oleandrin work as sodium-potassium ATPase inhibitors, which means that they interfere with the balance of ions inside cells. The muscle cells of the heart are particularly vulnerable to changes in sodium concentration, because sodium concentration is coupled to calcium export, and the calcium concentration inside the muscle cell is what regulates how strongly or quickly the muscle cell can contract. When there’s too much sodium, the cell can’t efficiently export calcium, and the cell contracts too strongly as a result. Erratic muscle contractions are certainly bad news for a healthy heart.
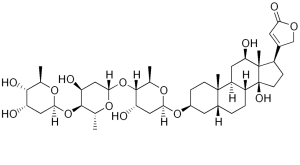
Digoxin: sometimes a help and sometimes a hindrance to heart function.
Unlike oleandrin however, digoxin has considerable utility as a medicine: the same calcium ion hoarding effect that’s so dangerous in a healthy person can be used to combat heart failure by promoting stronger contractions in a damaged heart, and digoxin gained FDA approval as a treatment for chronic heart failure and some kinds of heart arrythmias in 1998.
The initial use of digoxin came before beta-blockers were used to manage heart failure (HF), and there is ongoing study as to whether digoxin remains valuable as an HF management strategy in concert with other therapies. A recent article in the International Journal of Cardiology has undertaken a multivariable regression approach to attempt to classify which categories of patients are more likely to suffer higher mortality or further hospitalizations for heart failure following digoxin use. Their meta-study combined cases of over 7000 patients, and found that higher mortality and hospitalizations for heart failure were correlated with groups of patients that were female and had high blood pressure. Studies like this one may help identify which groups of patients can still benefit from digoxin and which groups should avoid it.
Extra nitty-gritty II: Digoxigenin as molecular label
Apart from its cardiac glycosides, Digitalis also harbors a supremely handy steroid, Digoxigenin (DIG), which I use routinely to label RNA molecules. Digoxigenin is a fairly small little molecule that can be coupled to the nucleotides that make up DNA or RNA (nucleotides=letters: A,G,T/U, and C), and there are specific antibodies for DIG that can be used to detect it anywhere it’s bound in a cell.

DIG-UTP. This labeled "U" is incorporated into RNA molecules just like regular UTP.
So when I want to find which cells in my tissue sample are making a certain RNA, I can make a probe with a complementary sequence and some of the U’s labeled with DIG. Then I can use anti-DIG antibodies (conjugated to an enzyme that makes a purple color under the right conditions) to look for the probe, with a technique called in situ hybridization.
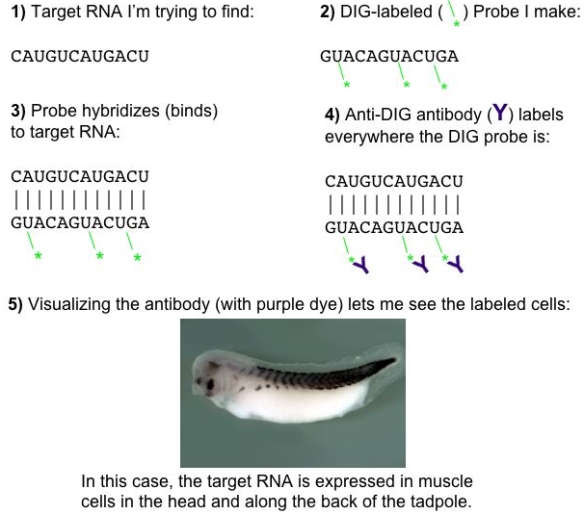
A little how-to for using DIG-labeled UTP to find a target RNA by in situ hybridization.
Want more details? Here are references for the articles mentioned:
Ather S, Peterson LE, Divakaran VG, Deswal A, Ramasubbu K, Giorgberidze I, Blaustein A, Wehrens XH, Mann DL, & Bozkurt B (2011). Digoxin treatment in heart failure – unveiling risk by cluster analysis of DIG data. International journal of cardiology, 150 (3), 264-9 PMID: 20471706
Lin, C., Yang, C., Phua, D., Deng, J., & Lu, L. (2010). An Outbreak of Foxglove Leaf Poisoning Journal of the Chinese Medical Association, 73 (2), 97-100 DOI: 10.1016/S1726-4901(10)70009-5